Welcome to Jun BMI Computational Lab!
We’re a research group at University of Cincinnati Computer Science Department, and we're using artificial intelligence to push the boundaries of healthcare. Our work dives into AI-powered immune checkpoint inhibitor discovery, analyzing genomic data, and figuring out better ways to spot potential treatment targets. We’re also working on improving how we detect cancer through smarter medical image analysis. In the big picture, we're aiming to help make smart healthcare more personal, more effective, and more accessible for everyone.

Teams

Assistant Professor
Dr. Jun Bai is an assistant professor at Computer Science Department at University of Cincinnati. She is PI of Jun BIMI Computaional Lab.

Po-Yu is a Ph.D. student at University of Cincinnati Computer Science Department. Po-Yu’s project focuses on developing innovative computational methods to design and generate the structures and sequences of immune checkpoint inhibitors—paving the way for more effective cancer immunotherapies.

Wei is a Ph.D. student at University of Cincinnati Computer Science Department. Wei’s project is developing new computaional methods to pinpoint promising therapeutic targets by combining insights from multiple types of genomic data—opening the door to more precise and personalized treatments.

Jordan is a master student at University of Cincinnati Computer Science Department. Jordan’s project focuses on Reinforcement-learning–based decentralized medical image analysis optimizes local models across multiple institutions through reward-driven updates, improving global diagnostic performance while preserving privacy and avoiding central data pooling.

Austin is a master student at University of Cincinnati Computer Science Department. Austin’s project focuses on graph-based generative AI framework that models characters as interconnected shape and attribute graphs to automatically create diverse, novel animation characters.

Anthony is a master student at University of Cincinnati Computer Science Department. Anthony’s project focuses on Quantum Machine Learning in Medical Image Analysis.

Nanini is a master student at University of Cincinnati Computer Science Department. Nandini’s project focuses on binding affinity esitmation.
News
- August 2025Congratulations to Wei and Po-Yu! Their paper, "DeepPH : A Multimodal Deep Learning Model for Predicting Enzyme Optimal pH Range", has been accepted to publish in the proceddings of ACM Conference on Bioinformatics, Computational Biology, and Health Informatics (ACM BCB).
- August 2025Congratulations to Po-Yu! His paper, “E(3)-Invariant Diffusion Model for Pocket-Aware Peptide Generation,” has been published in the proceedings of the International Symposium on Bioinformatics Research and Applications (ISBRA).
- June 2025Congratulations to Dang! He has received the AI Bearcat Grant for his project on medical image analysis using AI.
2025
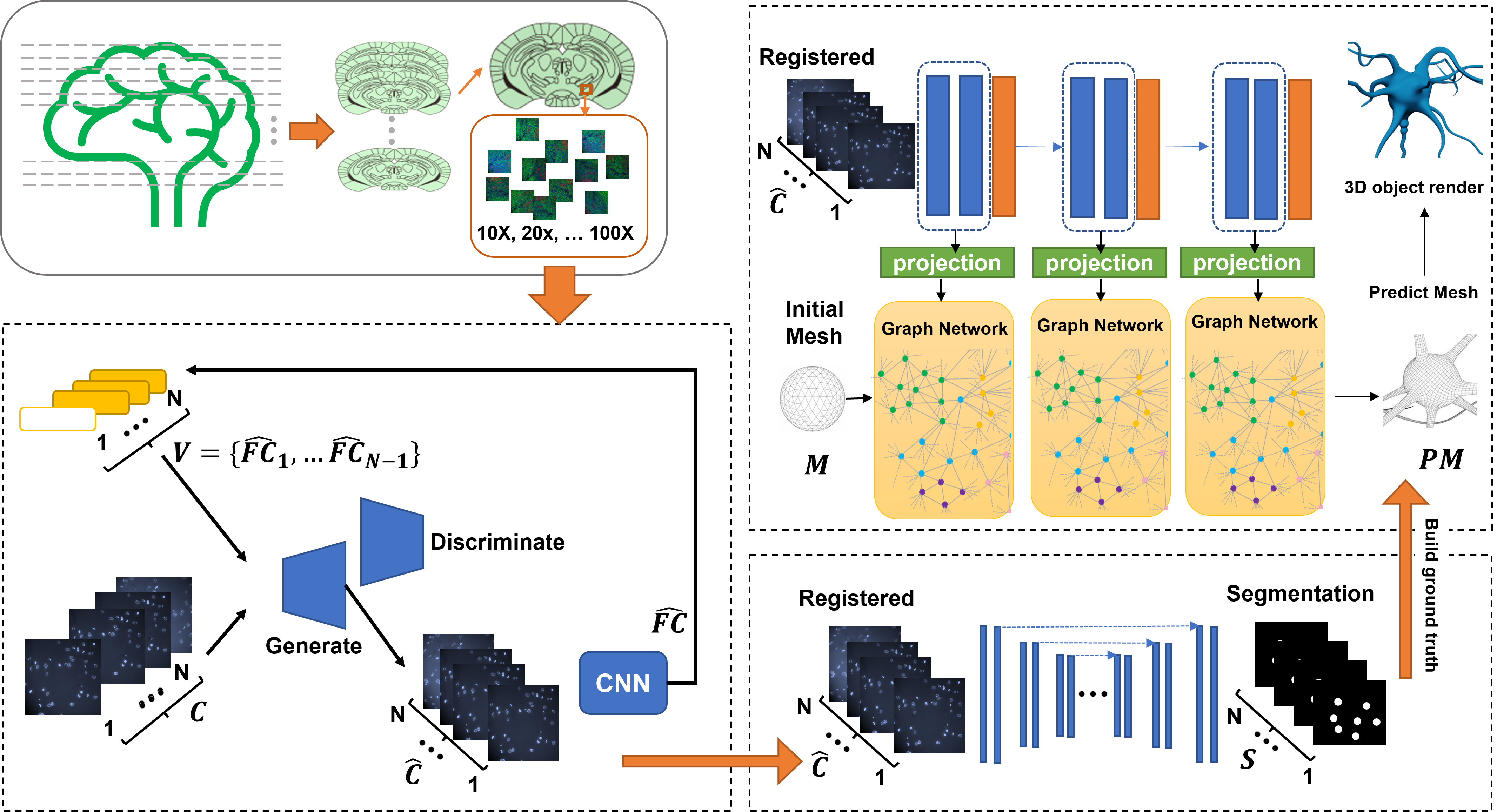
Ultraplex Microscopy image reconstruction App development
The challenge of unraveling the mysterious interplay between individual cells and intricate neural circuits is a daunting task, owing to its overwhelming complexity. However, the current project endeavors to surmount this challenge by creating a state-of-the-art software tool that is capable of seamlessly processing, annotating, registering, analyzing, and reconstructing 3D cells and neural systems with unparalleled precision. This revolutionary tool will serve as the cornerstone for the application of deep learning models and will pave the way for a deeper understanding of the intricacies of the biological world.
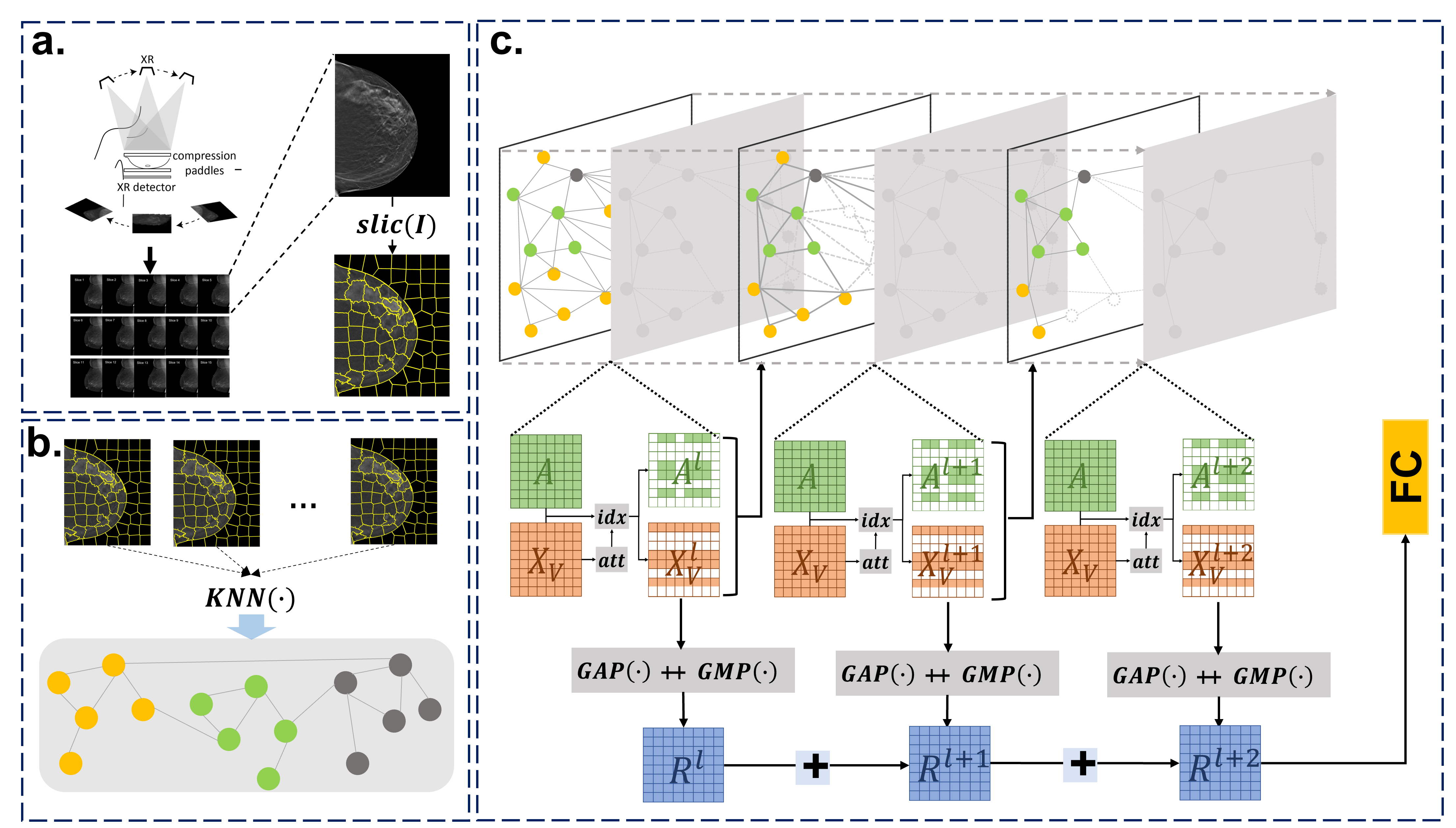
Digital Breast Tomosynthesis cancer prediction and localization using Graph Neural Network
The advent of digital breast tomosynthesis has transformed the landscape of breast imaging diagnosis, thrusting it to unprecedented levels of precision and accuracy. However, despite these remarkable advancements, the automatic detection of breast cancer using the high-resolution, high-volume, and complex images generated by digital breast tomosynthesis has remained a formidable challenge. To conquer this challenge, our research team has embarked on a pioneering journey of developing a revolutionary model for the detection of cancerous 3D mammogram images. In this groundbreaking study, we have developed an innovative model for more precise detection of cancerous 3D mammogram images. This novel model firstly represents 3D mammograms as graphs, then uses a self-attention graph convolutional neural network model to effectively and efficiently learn the features of 3D mammograms, and finally, by utilizing the extracted features, identifies the cancerous 3D mammograms with unparalleled accuracy. This proposed model will undoubtedly pave the way for more efficient and effective breast cancer diagnosis, and a major step forward in the fight against this devastating disease.
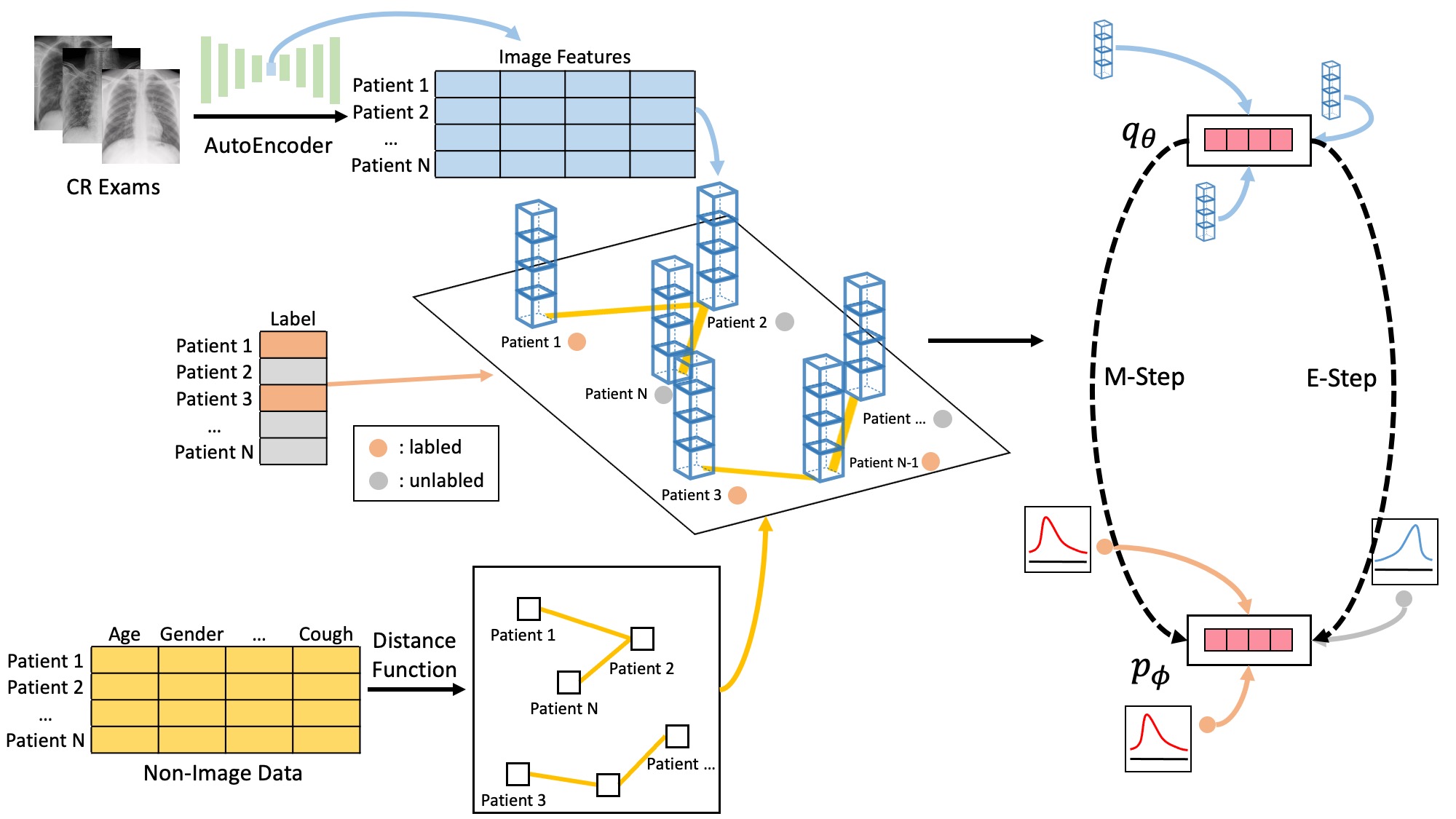
Semi-supervised classification of disease prognosis using CR images with clinical data structured graph
The rapid expansion of global connectivity and urbanization has created a perfect storm for the spread of disease on a worldwide scale. The outbreak of the SARS-COV-2 pandemic has placed immense strain on healthcare systems, particularly in the area of intensive care units. In light of this dire situation, the accurate prognosis of patients' need for intensive care units at the earliest stage of hospital admission has become a top priority, in order to ensure efficient allocation of resources. To this end, we have proposed a groundbreaking clinical data structured graph Markov neural network embedding with computed radiography exam features (CGMNN) to predict the intensive care units demand for COVID-19 patients. This innovative approach utilizes patient chest radiography and clinical data collected at the early stages of hospitalization to make more accurate diagnoses, ensuring that patients receive the care they need in a timely and efficient manner.
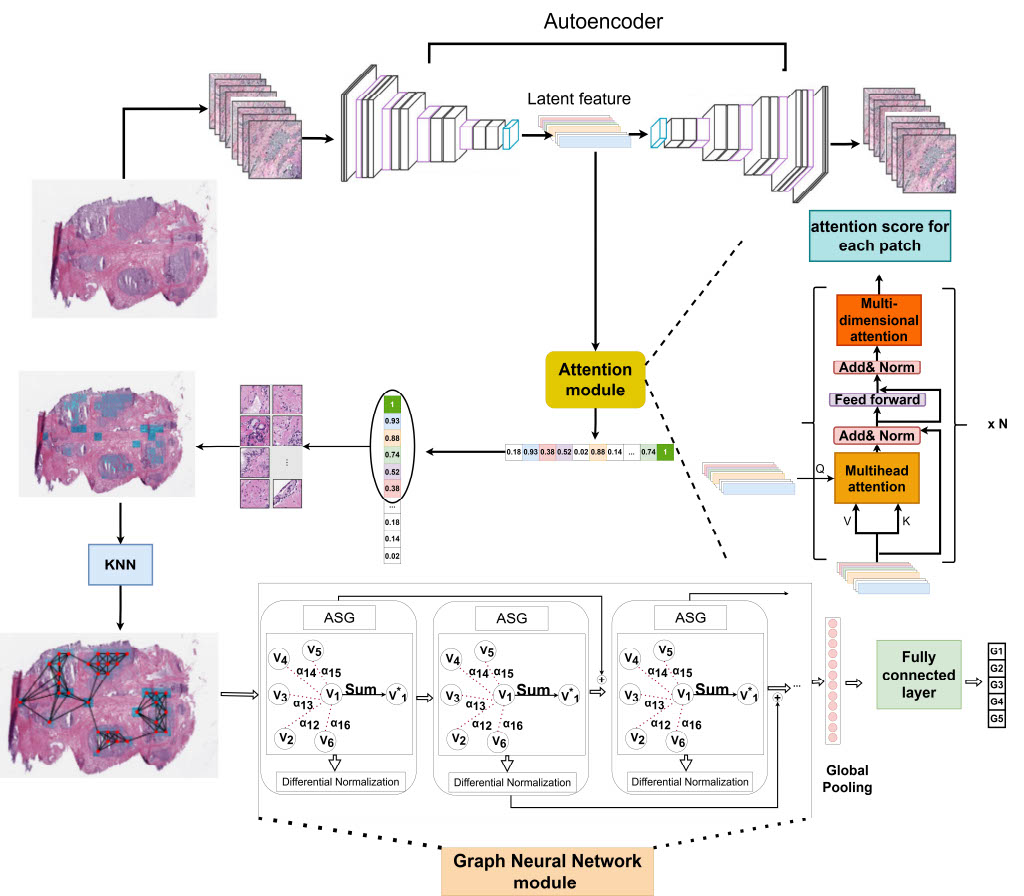
Weakly-Supervised Deep Learning Model for Prostate Cancer Diagnosis and Gleason Grading of Histopathology Images
Prostate cancer is a global scourge, afflicting men worldwide and ranking as the second leading cause of cancer death in the United States. One of the key prognostic features in prostate cancer is the Gleason grading of histopathology images. This grading system, which is based on the tumor architecture of Hematoxylin and Eosin (H&E) stained whole slide images (WSI) is assigned by pathologists and is known to be both time-consuming and prone to inter-observer variability. In recent years, deep learning algorithms have been employed to analyze histopathology images, with promising results in the grading of prostate cancer. However, these algorithms rely on fully annotated datasets, which are both costly and time-consuming to generate. In this work, we propose a novel weakly-supervised algorithm to classify prostate cancer grades. The proposed algorithm is composed of three steps: (1) extracting discriminative areas in a histopathology image by utilizing the Multiple Instance Learning (MIL) algorithm based on Transformers, (2) representing the image by constructing a graph using the discriminative patches, and (3) classifying the image into its Gleason grades by developing a Graph Convolutional Neural Network (GCN) based on the gated attention mechanism. This innovative algorithm is set to revolutionize the field of prostate cancer diagnosis and pave the way for more accurate, efficient, and cost-effective treatment of this devastating disease.
Molecular dynamics simulation to uncover the mechanisms of protein instability during freezing
Freezing is a widely used technique in the pharmaceutical industry for the preservation and transportation of biotherapeutics. In order to gain a deeper understanding of the mechanisms and molecular details of protein destabilization upon freezing, multi-scale molecular dynamics simulations of Lactate dehydrogenase (LDH) protein in phosphate buffer with/without ice formation were performed. The simulations were conducted under both fast and slow ice-growing conditions at 243 K, from one or two sides of the simulation box, respectively. The results showed that the rate of ice formation at all-atom simulations was crucial to the stability of LDH, as faster freezing rates resulted in enhanced structural stability and a higher number of intramolecular hydrogen bonds, less flexible protein residues, lower solvent accessibility, and greater structural compactness. Additionally, protein aggregation was investigated through coarse-grained simulations and was found to be initiated by extended protein structures and retained by electrostatic interactions of salt bridges between charged residues and hydrogen bonds between polar residues of the protein. Lastly, the study of the free energy of dissociation through steered molecular dynamics simulation revealed that LDH was destabilized by the solvation of the hydrophobic core and the loss of hydrophobic interactions. This study, for the first time, provides experimentally validated molecular simulations that reveal the detailed mechanisms of LDH destabilization upon ice formation and cryoconcentration of solutes, and it will help to improve the preservation and transportation of biotherapeutics.